FDA now accepts the Codex Definition of Kava Consumption.

The classification and regulatory stance of the U.S. Food and Drug Administration (FDA) on Kava has evolved significantly over the years. Kava, scientifically known as Piper methysticum, has been traditionally used by Pacific Islanders for its sedative and anxiolytic effects. In the United States, Kava has gained popularity as a dietary supplement, particularly for its potential to relieve restlessness and stress. However, its journey through FDA regulations reflects a careful approach to its safety and classification, finally ending in a contemporary view that recognizes Kava, when mixed with water, officially as a food.
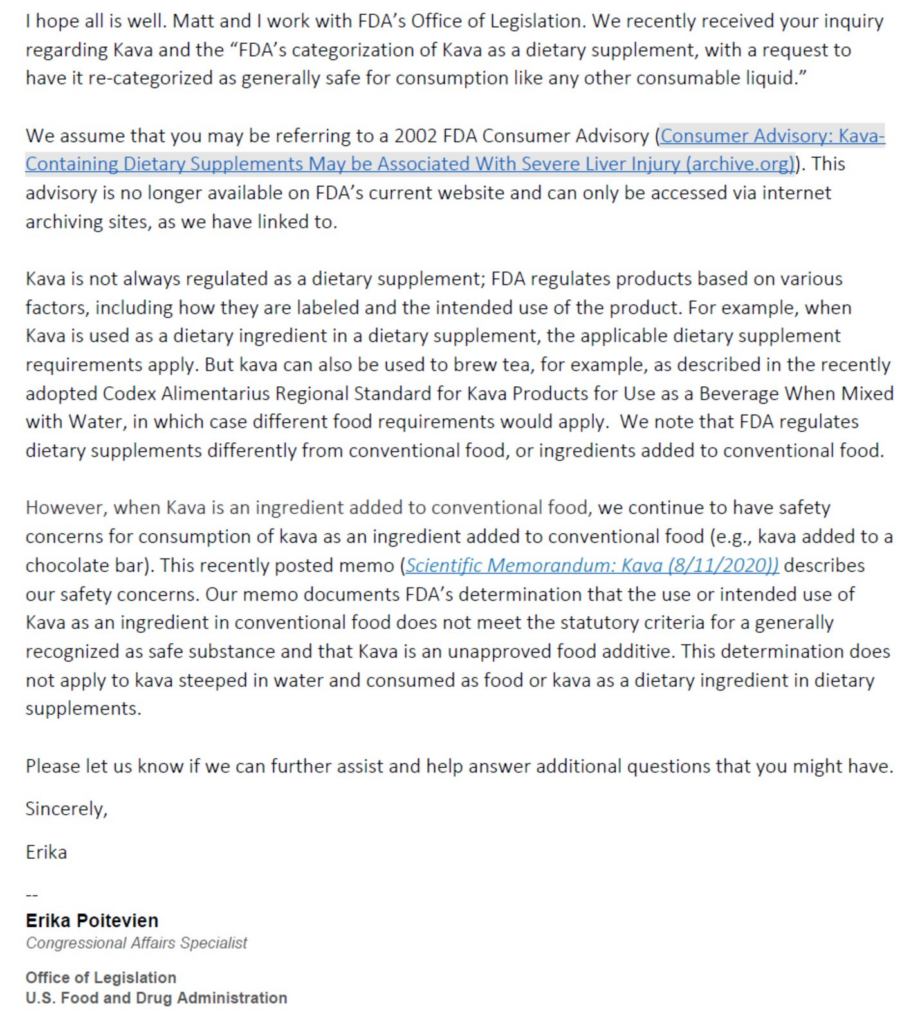
Historically, the FDA’s relationship with Kava was marked by caution and nuance, primarily due to concerns over its safety profile. In 2002, the FDA issued a Consumer Advisory warning about the potential risk of severe liver injury associated with Kava-containing dietary supplements. This advisory, now accessible only through internet archiving sites, signified a period of heightened scrutiny and caution towards Kava consumption, especially in forms that were concentrated or extracted. Also this document contained adverse event reports that have been discredited by the German Government as of 2015.
The FDA’s regulatory framework is intricate, with products classified based on various factors including labeling, intended use, and composition. For Kava, this meant a divergence in its regulatory approach. As a dietary ingredient in dietary supplements, Kava was subject to the stringent requirements that govern these products. These requirements can be as difficult to meet as for pharmaceutical preparations in some regards. This classification underscored the agency’s commitment to ensuring the safety and efficacy of dietary supplements available to consumers.
However, the narrative began to shift with the recognition of traditional and cultural uses of Kava that posed minimal risk when consumed in traditional manners. The adoption of the Codex Alimentarius Regional Standard for Kava Products for Use as a Beverage When Mixed with Water marked a pivotal moment in the reevaluation of Kava’s classification. This standard, which highlights the traditional practice of preparing Kava, paved the way for a more accommodating regulatory perspective, acknowledging Kava’s cultural significance and its safe use in a specific context.
The FDA’s recent communications further shed light on this evolved stance. In a detailed memo (attached below), the agency delineated its safety concerns for Kava as an ingredient in conventional food, reiterating that it does not meet the criteria for a generally recognized as safe (GRAS) substance when added to conventional food items, such as chocolate bars. This distinction highlights the FDA’s nuanced understanding of Kava’s risk profile, differentiating between its use in conventional food products and more traditional or dietary supplement contexts.
Crucially, the FDA’s current view officially recognizes kava mixed with water as a food, distinguishing it from its categorization as a dietary supplement or an unapproved food additive. This classification aligns with traditional consumption practices and acknowledges the minimal risk associated with such use. It represents a significant step towards embracing cultural practices and providing a regulatory framework that accommodates the consumption of kava in a manner consistent with its traditional use.
In conclusion, the FDA’s classification of Kava has undergone significant evolution, reflecting a careful consideration of both scientific evidence and cultural practices. The recognition of Kava, when mixed with water, as a food, not only demonstrates a respect for traditional consumption methods but also underscores the FDA’s commitment to public health and safety. By differentiating between various forms and uses of Kava, the FDA has sought to navigate the complex landscape of dietary supplements and food safety, ultimately providing a path that respects tradition while ensuring consumer protection.
FDA Email Below was attained with the help of Ed Johnston, a founding member of the IKO, and Member of the Association for Hawaiian ‘Awa (Dated September, 1 2023):